Join us / Join as audit team
Strategic Plan of Healthy Ecosystem for Traditional Medicine Clinical Trial Registration
ITMCTR aims to cooperate with experts, scholars and academic organizations in the field, provide dedicated channels for traditional medicine registration, establish clinical research registration standards, promote the openness and transparency of traditional medicine clinical research, help the high-quality output of traditional medicine evidence, and promote traditional medicine to better serve global health.
The Strategic Plan of Healthy Ecosystem for Traditional Medicine Clinical Trial Registration is proposed by ITMCTR, which tries to establish a clinical trial registration collaboration in traditional medicine to improve the publicity and increase the efficiency of ITMCTR. At the same time, it will promote the improvement of the registration quality of clinical trials in the field of traditional medicine, thereby promoting the high-quality development of clinical trials in traditional medicine.
Collaboration Model
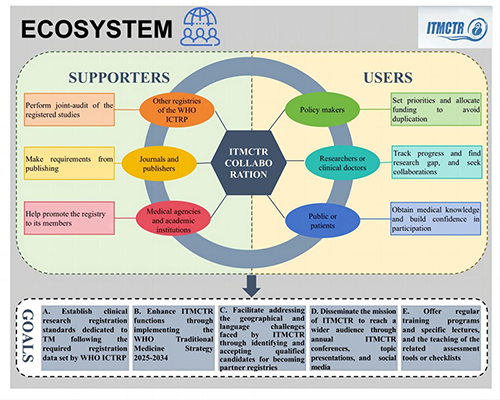
1. Collaboration with WHO Primary Registration PlatformsObjective: Establish an international coordination mechanism to enhance the global visibility and credibility of traditional medicine clinical trial registration.
Collaboration Methods:
· Jointly develop and improve registration standards for traditional medicine clinical trials to enhance data integrity, transparency, and usability.
· Conduct joint review of traditional medicine clinical trial registration projects to improve efficiency and streamline registration procedures.
· Facilitate data sharing, promoting global regulatory coordination and information exchange for traditional medicine clinical trials.
2. Collaboration with Journals and Publishers
Objective: Make registration a prerequisite for publication, improving the traceability and credibility of traditional medicine research.
Collaboration Methods:
· Require submission of a registration number in journal submission guidelines.
· Link registration with publication, ensuring that registered studies disclose results completely, avoiding selective reporting and publication bias.
· Organize academic workshops to educate researchers on the importance of trial registration.
3. Collaboration with Media and Academic Organizations
Objective: Increase ITMCTR’s visibility, expand its influence, and promote trial registration recognition among academia and the public.
Collaboration Methods:
· Raise public awareness of the value and significance of traditional medicine clinical trial registration through media campaigns.
· Partner with academic societies, research institutions, and industry associations to promote ITMCTR registration via websites or newsletters.
· Host international forums to encourage cross-disciplinary and cross-regional collaboration and exchange.
Service Model
1. Services for PolicymakersObjective: Provide accurate data support to aid scientific decision-making and optimize resource allocation.
Services Provided:
· Industry analysis reports to identify research priorities and support policymaking.
· A data-sharing platform to help governments and funding agencies monitor research trends, avoid redundant studies, and enhance funding efficiency.
· Regular policy forums offering expert insights to ensure policies align with international trends and clinical needs.
2. Services for Researchers and Clinicians
Objective: Provide an efficient registration platform and data services to enhance research quality and impact.
Services Provided:
· Online registration system: Simplifies registration, offers multilingual support, and improves global accessibility.
· Trial tracking tools: Allow researchers to update and manage trial information, forming comprehensive study records.
· Data analysis support: Helps researchers identify research gaps, optimize trial design, and improve study quality.
· Interdisciplinary collaboration platform: Facilitates networking and cooperation among researchers worldwide.
Services for the Public and Patients
Objective: Provide transparent trial information to enhance public trust and patient engagement in traditional medicine research.
Services Provided:
· Public information portal to facilitate trial information access and improve transparency.
· Patient engagement support, including educational materials to help patients understand clinical trials and improve recruitment.
· Feedback mechanisms allowing patients and the public to express needs and concerns, enhancing the clinical value of research.
Future Plan
As stated in the goals of the Strategic Plan, ITMCTR will make all the necessary efforts in the future to:
· Establish clinical research registration standards dedicated to TM following the required registration data set by WHO ICTRP and highlighting the specific characteristics of TM.
· Enhance ITMCTR functions through implementing the WHO Traditional Medicine Strategy 2025–2034 focusing on strengthening evidence base and evidence-informed decision making for TM.
· Facilitate addressing the geographical and language challenges faced by ITMCTR through identifying and accepting qualified candidates for becoming partner registries of ITMCTR following WHO rules and procedures.
· Disseminate the mission of ITMCTR to reach a wider audience such as organizing annual ITMCTR conferences, making topic presentations or activities in academic conferences, and using social media.
· Offer regular training programs and specific lectures regarding the general introduction of requirements to guide filling in each registration item, as well as the teaching of the related assessment tools or checklists.
Join the Strategic Plan
You are welcomed to join the Strategic Plan of Healthy Ecosystem for Traditional Medicine Clinical Trial Registration!
Contact us at:itmctr@ccebtcm.org.cn